Ready to implement assays for preclinical and clinical testing
During drug development, creating and validating de novo assays for phase-appropriate use takes a significant amount of time. Having off-the-shelf assays in place and ready to use for regulated or nonregulated sample analysis directly translates into faster timelines and reduced costs, two critical aspects of designing successful preclinical and/or clinical studies.

BioAgilytix offers ready to implement solutions that matter
At BioAgilytix, we know any delays in preclinical and clinical testing can have a big impact on the success of a drug program. Therefore, we have invested our own resources into developing and validating assays in-house so that we can offer a broad range of off-the-shelf solutions to our clients. These offerings cover a variety of assay types from our extensive off-the-shelf biomarker selections to anti-drug antibody (ADA), neutralizing anti-drug antibody (NAb) assays and more.
View Our Off-the-Shelf Assay Menu
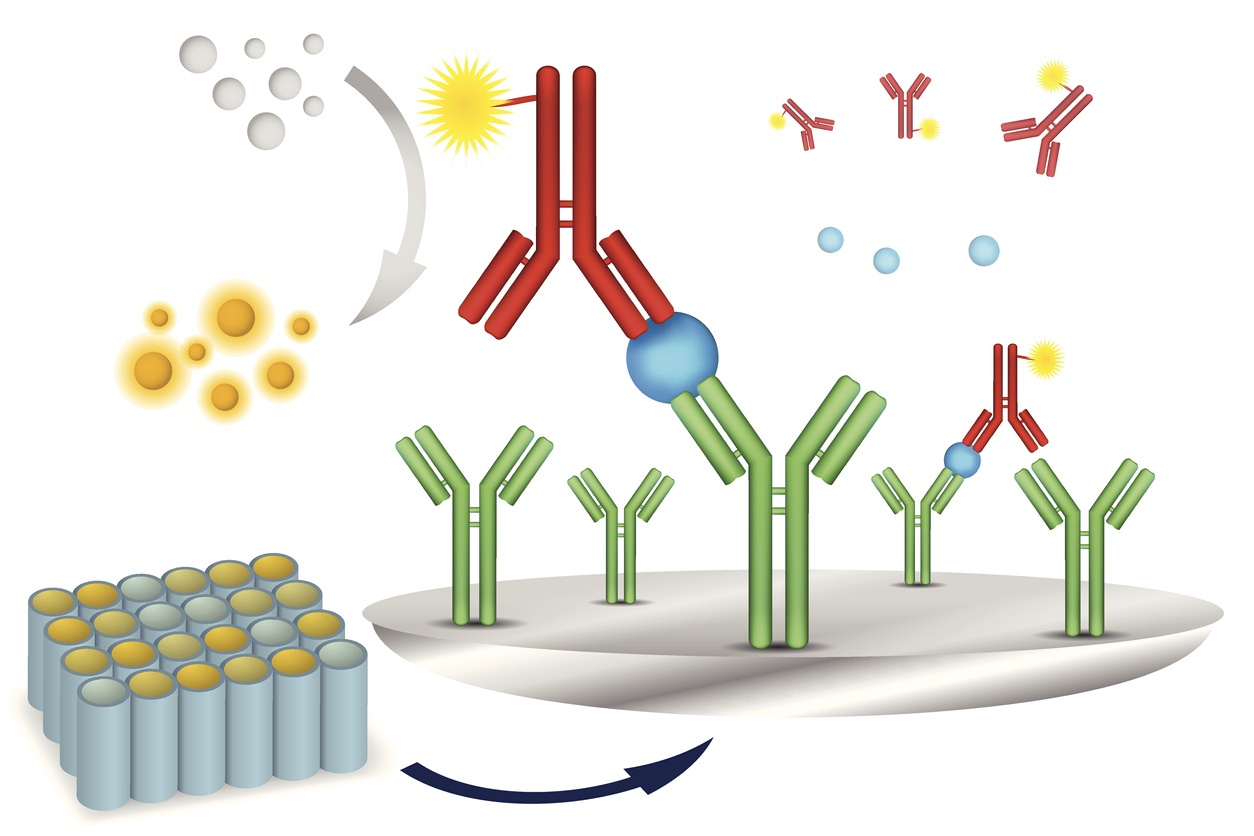
Our growing selection of off-the-shelf options for your bioanalytical needs
Our clients need quality sample analysis results quickly and efficiently. Our off-the-shelf assays help make this possible. BioAgilytix has a long history of putting the needs of our clients at the front of what we do and investing in off-the-shelf assays is no exception. We continue to look for new assays to bring in-house as part of this effort and are proud to offer a range of off-the-shelf assays including but not limited to biomarker, pharmacokinetic (PK), humoral immunogenicity, cellular immunogenicity, and molecular assays in support of regulated or non-regulated, preclinical or clinical sample analysis.
An expansive off-the-shelf biomarker menu
BioAgilytix has long been known as an industry leader in biomarker work. Our expertise includes a wide range of disease areas including oncology, metabolic, CNS, inflammatory, and infectious disease and matrices including serum, plasma, and tissue homogenates. The biomarker menu on our website is an easy-to-use resource for clients searching for a specific assay. This off-the-shelf listing includes assays across a variety of commonly used platforms including but not limited to:
- ELISA
- MSD-ECL
- Gyros
- ProteinSimple
- Luminex/Bioplex
Immunogenicity offerings
As an experienced CRO provider, we know that although each therapeutic is unique, there are often common bioanalytical strategies to supporting multiple different drug programs. For example, advanced therapeutic modalities such as adeno-associated virus (AAV) gene therapies typically require ADA and NAb assays specific to the AAV viral capsids. BioAgilytix has a wide selection of preclinical and clinical AAV ADA and NAb assays available off-the–shelf. We have taken additional steps to ensure that we can provide a comprehensive bioanalytical support package by offering validated off-the-shelf ELISpot assays for these programs as well.
Nonproprietary small molecule assays
As one of the largest bioanalytical LC/MS service providers in the U.S., BioAgilytix has developed more than 2,400 proprietary and non-proprietary methods, including many validated and ready-to-validate non-proprietary assays for small molecule bioanalysis. These are ready to quickly implement as part of our off-the-shelf assay strategy to help cut time and costs for your small molecule program.
Specialty off-the-shelf solutions
In addition to the larger categories of off-the-shelf assays, BioAgilytix has carefully selected a number of specialized assays to validate in-house to meet our customers’ needs. These include:
- Flow cytometry panels
- PCR based methods such as RCL
- Anti-PEG immunoassays
- PK assays for commonly used co-medications

How BioAgilytix utilizes off-the-shelf solutions
BioAgilytix take our commitments to our customers seriously. Your success is our success and that means meeting timelines and delivering high quality, cost-effective bioanalytical solutions. Our off-the-shelf menu means that you have access to already validated, ready to implement assays when you need them. We provide an upfront summary of performance and full access to our regulatory-compliant method validation reports, allowing you to go straight to sample analysis while remaining fully compliant with the applicable regulatory documentation required for your preclinical or clinical program.